
Ningbo Inhal Pharma Co., Ltd., led by Professor Jingxu Zhu, academician of the Academy of Science of the Royal Society of Canada and Canadian Academy of Engineering, is located in Ningbo National High-tech Zone, Zhejiang Province. Inhal is a scientific and technological innovation company specializing in the research and development of inhalation formulation, inhalers, and filling equipment. Inhal is committed to creating a precise drug delivery platform for integrated drug delivery, realizing interconnection with upstream-downstream enterprises, and providing turnkey solution in the field of inhalation drug delivery for global pharmaceutical companies.
Academician Zhu's team has been engaged in inhalation drug delivery research since 1994 and has accumulated more than 20 years’ experience. Inhal has achieved drug screening and evaluation, formulation design and optimization of powder aerosolization, research and development of dry powder inhalation device, precise and quantitative repackaging technology and equipment development of ultrafine powder drugs, and construction of lung targeted drug delivery system.
The team has three core technologies: ultrafine powder preparation and fluidization technology, ultrafine dry powder drug quantitative repackaging technology (200μg±5%), and low-resistance inhalation flow channel design, development, and verification technology. Furthermore, the team has filed 18 invention patents in China, the United States, Canada and Europe. In the field of inhalation device development, the team has developed various products, such as Breezhaler/Elpenhaler protected by new appearance patents, Turbuhaler, and blister inhaler.
Inhal’s interdisciplinary team consists of pharmacy, chemical engineering, pharmaceutical engineering, industrial design and industry veteran, including 1 academician, 1 senior research engineer, 6 doctors, 9 masters, who have rich experience in product development and commercialization, and will continue to bring innovative products to the market.

Cooperated with GSK to develop a new type of ultrafine powder filling fluidized bed equipment

Developed a quantitative filling equipment for ultra-fine powder : industrialized rotatory fluidized bed

Developed a series of blister dry powder inhaler independently, which can deliver pure powder without auxiliary materials

DPI was used to deliver exogenous surfactants to the lungs for the treatment of lung injury caused by mechanical ventilation
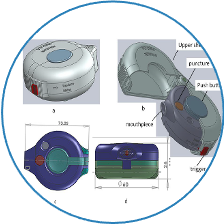
Independently developed a blister-based dry powder Inhaler(Inhaler WU2011,Carrier-based formulation)
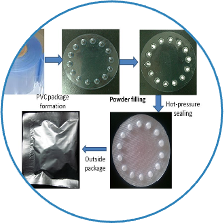
Completed the pharmaceutical study of budesonide formoterol and the prescription study of salbutamol sulfate
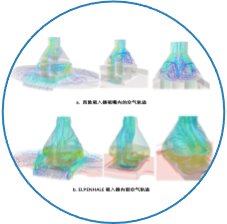
Using amino acids as model drugs to establish a screening platform for delivery of dry powder inhalation(pulmonary delivery of biopharmaceuticals, vaccines, etc.)




